Why Does a Pure Substance Always Have the Same Density
Unlike the situation with water there is no maximum density point. Due to crystalline structure all atom and molecule are arranged in a well defined manner unlike liquids.
Does The Shape Size Or Temperature Of Matter Affect Its Density Ms Shon S Spectacular Science
I assume that different conditions must include different temperatures and pressures so the answer is no Lets take pure.
. These substances mainly have a constant or uniform composition throughout. Examples of Pure Substances. Also correct assuming that the definition of unified atomic mass units amu remained the same.
Any sample of iron is 100 iron. Because a pure substance indicates that it is exactly that a substance. But hardly ever does quartz contain even trace amounts of aluminum.
Compound will always have the same melting point. Students make a graph of the relationship between the volume and the mass of water. 3 You are given a square piece of aluminum foil and asked to calculate its density.
The density of a substance is always the same at a given temperature and pressure. Two similar-looking substances are burned. Volume and mass are extensive properties.
Phases of a Pure Substance A pure substance may exist in different phases. Another characteristic of properties is that the value of a property at the present is not dependent on the history of the substance. Liquid you might consider that Carbon C and Helium He as such a substances.
Most other pure liquids are like ethanol in this respect. Density is something which is defined by parameters mass and volume. Answer 1 of 2.
A cup of water cannot have the same mass as a drop of water. Common units for density are gcm3 gmL kgm3 and kgL. A pure substance usually participates in a chemical reaction to form predictable products.
A pure substance is homogeneous that is has uniform composition throughout the whole sample. First the presence of impurities will result in a lowering of the melting point. Solutions show the typical behavior of the pure liquid with temperature but the density is also strongly affected by the quantity of dissolved material.
A pure substance does not have to be of a single element or compound. Does a pure substance always have the same density when measured under different conditions. Density is very slightly less than specific gravity.
We can see that the density decreases with temperature through this range. Specific gravity is the ratio of the density of a substance to the density of a standard usually for a liquid or solid and air for gas. Even though there is more molasses mass in test tube A the molasses also takes up more space volume.
Even if you cut the object into a million pieces they would still each have the same density. One produces a red flame and the other produces a yellow flame. Ratio of the density of the substance to the density of the reference substance which is usually water at the same temp.
This means that regardless of the objects shape size or quantity the density of that substance will always be the same. Solids have a high density than the liquids because of their crystalline structure. Density is simply the mass divided by volume.
Specific gravity is a ratio of two densities so it has no units. When can they not be used. Second an impure compound will exhibit a wider melting point range.
Now if somehow quartz had the same look and smell as aluminum I doubt you could tell the. Using Density to ID Substances Each substance has a density that differs from the densities of other substances. A sample of the element iron Fe is composed only of iron atoms so its composition is said to be uniform throughout the sample.
Martins answer is correct but we can also arrive at the same conclusion using a simple dimensional analysis approach. This means that it is the amount of the substance in a specific unit of space. Explain in detail how you would do this including in your explanation the data you would need to.
2 Can density be used to identify unknown substances in lab. For example quartz and aluminum at least in this table have the same density. Density is not a particularly good indicator of what a substance is.
Pure substances are mostly homogeneous in nature containing only one type of atoms or molecules. Density is an intensive property. The mass and volume of a substance is directly proportional to the amount of matter making up the substance.
This is an example of a _____ test. The reason that the molar concentration is often called constant is twofold. However because the density of pure water is so close to 1 09976 grams per cubic centimeter specific gravity and density are nearly the same value so long as the density is given in gcc.
Students measure the volume and mass of water to determine its density. The density of a substance is the same regardless of the size of the sample. The density of liquids and solids has a much weaker dependence on the temperature than gases do therefore it can be regarded as approximately constant.
For example pure benzoic acid has a melting point range of 121-123 C. The substances have fixed boiling and melting points. If it were 24 g instead of 12 g then the weight of 1 mole of substance would equal 2 times the atomicmolecular mass in grams.
Units for density consist of a mass unit divided by a volume unit. 1 Why does a pure substance always have the same density. Not really because the density varies already through thermal expansion.
A pure substance always has the ____ density when measured under the ____ conditions. If however the sample is not pure two phenomena will be observed. I actually disagree with the question.
But as there actually isnt any universal and exact definition for the difference of solid vs. That is because the density of liquids and solids is temperature and pressure dependent. The mass and volume both change when changing the amount of molasses.
However the density does not change. A mixture of two or more phases of a pure substance is still a pure substance as long as the chemical composition of all phases is the same. Because a pure substance indicates that it is exactly that a substance made of a specific combination of elements it will always have the same density because those elements can only take one.
This is because the mass and volume increase at the same rateproportion. Density is the ratio between mass and volume as mass increases the volume also increases in the same ratio so density for every pure substance at any given temperature is always constant. Then they measure the mass of different volumes of water and discover that the density is always the same.
Why or why not.

2 1 Classification Of Matter Pure Substance Has A Defined Composition And Cannot Be Separated Into Simpler Substances By Physical Means Each Substances Ppt Download
Can Substances That Are Made From The Same Material Have Different Density Even If They Have Different Mass Or Volume Quora

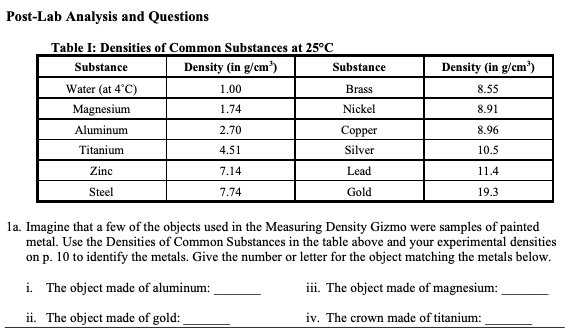
Solved Post Lab Analysis And Questions Table I Densities Of Chegg Com
Comments
Post a Comment